Calendar of Events
August 2017
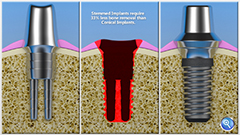
Announcement of the official release of the McMaster University live mammal implant study. The study compared adjacent bone cell ‘survival’ and growth between the Stemmed Implant (one-touch press) vs. the traditional conical implant (repeated torqueing ala ratcheting) as predicted by the S.I.T. CTO. The prediction by our CTO is a simple application of fracture mechanics and/or linear elasticity theory (known and understood by material engineers since the 1900s) applied to human bone tissue or osteoblasts. It is in fact common sense for any woodworker…however bone is live
The study concluded the following:
Initial observation of a dual-stemmed 3D printed dental implant has shown successful bone growth and bone-implant contact … new bone formation was noted in between the stems of the device…Conventional implants did have indications of stress cracking and show bone debris
In order to read more and understand the details of the study, please go to the Hindawi International Journal of Dentistry:
Hindawi International Journal
June 2017
Information session to update existing SIT investors on upcoming plan to re-assign ownership to better reward seed investors and put forth a plan to attract new investors….basically an increase in ownership for new investors compared to the crowd funding proposal.
February 2017
Board of Directors assignment and agreement on organizational and strategic structure. Previous Board member Martin J. Lococo and APJ Sheppard have been assigned to replace previous board members as a result of the Re-organization.
January 2017
Reorganization of the Board of Directors, following a reconsideration of crowdfunding efforts with a Las Vegas corporation, including the appointment of APJ Sheppard as the new President and CEO and Martin Lococo to oversee new investor relations
May 04, 2016
SIT president Christopher Ostrovski was invited by the Brazil-Canada Chamber of Commerce (based in Sao Paulo, Brazil ) to participate in a partnering seminar. As a result, several licensing and partnering opportunities are being investigated with companies in Brazil, Argentina, Chile, Uruguay and Brazil. The companies are of special interest because of the market potential and a regulatory regime that is easy to navigate.
May 28, 2015
SIT’s President & CEO makes presentation on Stemmed Implant Technology and its experience with Additive Manufacturing at the Life Science Ontario and BioLinc held at Brock University.
April 30, 2015
SIT participated in the first “ Canada Makes: Additive Manufacturing Forum “ Mohawk College, Hamilton Ontario, Canada.
April 08, 2015
SIT’s President and CEO participated in a by invitation only roundtable discussion with the Minister of Science and Technology the Honourable Ed Holder – on issues that industry is facing for development and growth, and if the role of government initiatives is helpful.
May 28-29, 2013
Bio Finance
St. Andrew’s Club & Conference Centre
150 King Street West, 27th Floor, Toronto, ON, M5H 1J9
June 27, 2012
Invitation to present at
the Life Sciences Ontario Meeting, Wednesday June 27th @7.30 AM,
Ryerson University
133 Mutual Street,
Toronto, ON.
May 29-30, 2012
Bio Finance
St. Andrew’s Club & Conference Centre
150 King Street West, 27th Floor, Toronto, ON,
M5H 1J9
May 23, 2012
Presentation, Life Sciences Ontario
BIO 2012, Boot Camp
Toronto, ON
April 17, 2012
Presentation at St. Catharines Club
St. Catharines, ON
May 28-29, 2011
Bio Finance
St. Andrew’s Club & Conference Centre
150 King Street West, 27th Floor, Toronto, ON,
M5H 1J9
Latest News
November 2017 Newsletter ...
- Release of McMaster animal study showing superior osseointegration of the Stemmed Implant device:
https://www.hindawi.com/journals/ijd/2017/5920714/ - Finalization of new corporate structure to ultimately benefit existing and new investment partners.
- After rejecting the crowd-funding and other investment offers, the BoD has finalized the 2018 investment strategy. SIT is looking to attract one major or two major investment partners that will help SIT attain both HC and FDA (and possibly international) regulatory approval. Previous investment “offers” have not committed to implement the technology (buyouts) and the BoD has also agreed to allow international investment/partnerships.
August 2017 Newsletter ...
- Announcement of the official release of the McMaster University live mammal implant study. The study compared adjacent bone cell ‘survival’ and growth between the Stemmed Implant (one-touch press) vs. the traditional conical implant (repeated torqueing ala ratcheting) as predicted by the S.I.T. CTO. The prediction by our CTO is a simple application of fracture mechanics and/or linear elasticity theory (known and understood by material engineers since the 1900s) applied to human bone tissue or osteoblasts. It is in fact common sense for any woodworkers…however bone is live.
January 2016 Newsletter ...
- SIT has entered into an agreement with The University of Texas, San Antonio – School of Dentistry to conduct and carry-out clinical trials for the SIT dental implant. Dr. Geza Terazhamly, Former Dean and Professor Emeritus of Dentistry, will be heading the trials.
November 2015 Newsletter ...
Shaping the Evolution of the Dental Implant Business- Stemmed Implant Technologies is about to undergo a significant change. In the coming months we will be moving from a small company with a great idea to a much larger company with the financing, corporate sophistication and marketing savvy to face the challenges of the commercial marketplace. While on the face of it our patented technology is simple, bringing it to market for use in the dental/medical community is not. The regulatory process is multi-faceted and obtaining the required financing in today’s climate is challenging. The journey this far has been thrilling, but I believe the real excitement lies just ahead. We will be challenged as we go through the approval process and refine the business case for both investors and end users, but I am confident that moving through the regulatory process will make us stronger and underscore the inherent advantage of our technology.
Widespread acceptance of our technology is inevitable. This newsletter details some of the watershed moments on our trip to this point and time, but more importantly, points to our directions for the future.
I invite you to join us as we embark on the next steps of our incredible journey and grow with Stemmed Implant Technology Inc. as it transforms into a significant player in this important niche area of dentistry and medicine.
Regards,
Chris Ostrovski
President
Stemmed Implant Technology Inc.
New Members of the SIT Team
In order for SIT to grow to the point where its technology is accredited and licensed internationally, the company requires new and specialize talent. To that end, the management team has been expanded to include:

-
Scott Proctor, Chief Operating Officer:
Mr. Proctor is a widely respected entrepreneur with professional experience in international accounting, finance and taxation. He has helped finance and launch numerous startup companies. Mr. Proctor is Principal of Australia Trading LLC, a company he formed in 2002 to provide expert advice to senior managers on subjects ranging from securities policy and regulation to the international financing. He has carried on business across the globe, at times managing multi-location international offices.
As SIT looks to international markets for clients and investors, Mr. Proctor’s experience and connections will be critically important. As SIT moves forward with a crowdfunding options, Mr. Proctor's strategic approach to successful offerings will position the company for success.
In addition to his experience, Mr. Proctor brings the securities and legal expertise of David LeGrand of Black & Lobello. Mr. LeGrand and Mr. Proctor have worked together on a succession of projects over the past 12 years. Black & Lobello will serve as security attorneys for SIT. The firms website is http://blacklobellolaw.com
- Dr. Allison Komiyama, Regulatory Coordinator:
Ms. Komiyama is a former Food and Drug Administration reviewer who has served as an expert in regulatory submissions. She will develop and manage the required regulatory process - from clinical trial administration to communication with government regulatory agencies, nationally and internationally. Her involvement will expedite the approval process by avoiding duplication of effort and ensuring procedures undertaken for one regulatory authority will be suitable to meet the standards of other jurisdictions.
Ms. Komiyama is the Principal of AcKnowledge Regulatory Strategies, a consultancy for biomedical manufacturer who work with implantable and other patient-contacting medical devices.
- Dr. Geza Terezhalmy, Clinical Trial Supervisor:
In order to provide appropriate evidence of the suitably of the Company’s technology, SIT will conduct clinical trials sufficient large enough to provide detailed information to accreditation authorities within the US Food and Drug Administration and Heath Canada. SIT has been fortunate to engage Dr. Geza Terezhalmy and his team with University of Texas (San Antonio), and the US Naval Research Station (Bethesda Maryland), to carry out the clinical trial. The number of people to be tested is unknown until the commencement of the clinical trial, but trials have commenced assuming at least 20 -25 different transplant patients will be required for treatment and post-treatment observation.
Dr. Terezhalmy is recognized globally for his clinical trial work. He is a supporter of our technology and is currently preparing an article for submission to peer reviewed journals detailing the value of SIT technology and it use in practice.
R&D Funding Update - Over the last few years the Company has met repeatedly with private medical funders and various venture capitalists funds. In almost every case, the funding party wanted the majority of the Company equity and large up front fees. After considerable time and effort, management has determined this is not an acceptable financing strategy. While other companies in the vertical may be prepared to sacrifice a majority of the equity, SIT managers believe there is another way forward.
In the last few year a promising new financial instrument has enter the marketplace. It is known as crowdfunding. It is anew, but tested alternative to fundraising in major markets. Companies such as SIT offer their shares directly to private investors through a regulated website, rather than through an exchange. The investment proposition is presented online through a crowdfunding platform, rather than thorough a stock brokerage firm. Crowdfunding allow small investors to invest directly in companies such as SIT that are in early development stage. The type of share an investor receives is determined by mutual agreement in advance of the sale, but it is different than those available through traditional investment opportunities. The risk involved is higher than in traditional markets, but the potential returns are significantly higher. Although this type of exchange is new, the securities laws, regulations, required disclosures, and risk to Company Directors, are identical and the same quality and completeness of information is required by law.
Are they successful? Not always, but at a recent medical device manufacturers and developers conference held in in San Diego, a full session focused on the challenges of obtaining regulatory approval of medical devices and financing examined the Crowdfunding option. As part of the discussion, case studies were presented on several companies that successfully raised $2,000,000 to $7,000,000. SIT management is carefully reviewing those case studies.
Moving Forward: Our Crowdfunding Plan:
To generate the financing required to take SIT from its current position, through the regulatory process and into international markets, the Company will need to present a series of crowdfunding offerings. One alternative under consideration is holding one round of crowdfunding for each step in the complex process of product certification and related clinical trials. In order to protect the value of the equity in the Company, it is management's intention to raise only the amount of capital required for each major step. As one goal is achieved, the Company will offer the required number of shares to fund the next step. As each step is completed, risk decreases and the price of shares being offered will be increased. Management believes this is the best approach to ensure that those who support the company receive the largest return on investment.
To make SIT shares as attractive as possible, the Company will open a US parent company that will acquire the shares of SIT Canada. The shares of all current shareholders will be exchanged automatically, and there are no income tax implications. In essence, nothing changes, except that the parent company will be registered in Nevada. This move will allow SIT to attract a wider and larger investor group and raise funds in US dollars. As most of the R&D costs and contracts are already in US dollars, management believes the geographic transition serves the best interest of the company and shareholders. Some investors will be pleased that the value of their investment has effectively been converted to US currency with no exchange cost.
Promissory Note Financing Ending :
Over the past several years the Company has accepted investor funds in various forms. Most recently the Company had offered Promissory Notes with interest bearing terms and convertible features. In order to conform with securities regulations, the Company will stop offering these notes prior to the crowdfunding documents being finalized and released. Management cannot accept any further notes after November 30, 2015. If you have a registered note commitment, this is the last day the Company can accept your funds.
Please understand the information available to investors is limited to newsletters and material found on the corporate website. All information to be released as part of the Crowdfunding offering is confidential and subject to securities laws that ensure all investors obtain the same information at the same time.
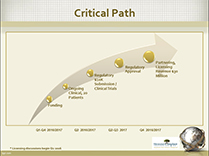
Our Regulatory Approval Plan - Our customers are worldwide, so our focus must be on identifying and working through the regulatory approval process that will lead to greatest international acceptance of our technology by the entities that license our technology. This will not be easy. The government product approval process is expensive and includes administrative measures, laboratory measures, animal testing and clinical trials. While the approval board in every country has the same safety objectives, their procedures and requirements often differ. Established standards often do not exist and applicants must deal with guidelines that can be open for interpretation.
At SIT, our strategy will consider the big picture first. Rather than focus on meeting the unique demands of a single regulator, we will proceed with the development of a high-level blend of of professional protocols that meet the requirements of Health Canada, the United States FDA and the major European approval bodies. Through careful management, and continual consultation with the regulators, we believe SIT will be able to carry out most tasks or test in a way that satisfies each of the relevant bodies, maximizing the use of resources while minimizing costs.
There is not always a clear path or route from the beginning to the end of the regulatory process, but by being flexible, and being prepared to make modifications based on the results of clinical trials, we can navigate this process successfully.
We have the greatest technology. Now we need to build the organization that can push for the required approvals and create the infrastructure that will make it available to professionals and the public. We also need to create a situation that makes it attractive to investors.
Clinical Trials: The Gateway to Licensing - Licensing the Company’s technology remains a key focus of the Company’s business plan. The level of interest in licensing this technology, and the amount a customers is willing to pay, rise dramatically following regulatory approval of the Company’s product, as well as with positive results of clinical trials. The pursuit of clinical trials is essential to regulatory approval and the Company is finalizing the arrangement with respect to its first clinical trial with Dr. Terezhalmy of the University of Texas. The trial will include the selection of patients, the placement of implants and monitoring of results. The process is scheduled to take 24 months to complete. We expect compelling data within the first year of the trials. The Regulatory approval process has already commenced with the engagement of Dr. Komiyama as Regulatory Coordinator. The company is ready do negotiate with serious licensing interests during latter stages of regulatory approval, and as clinical trial results are reported.
Medical Testing at McMaster University - Clinical testing involves using the Company’s dental implants on a group of people who need this procedure and agree to have their results monitored as part of the clinical trial process. In addition to clinical testing of people using dental implants, regulators require that the Company’s technology is used in other forms of experimental medical testing. A comparative (SIT implant vs. conventional implant ) animal study over time was conducted. The preliminary results show the bone tissue is bonding to the dental implant far better than the technology used in current dental practice. In the short term this is good for our regulatory approval of dental implant technology. In the long run it shows our technology may have a role in orthopedic surgery in the future.
Hip Replacement Implant?
- In cooperation with McMaster University a prototype of a hip replacement implant has been developed where the steel from an artificial hip that is joined to the bone has been modified using the Company’s patented technology More work needs to be done, but current animal test results at the university indicate SIT’s technology encourages earlier bone growth than traditional technology.
Major Milestones
- As the management of SIT we are focused on tomorrow and the next phases of growth. For the benefit of investors, and other interested parties, a detailed listing of our important milestones can be viewed by clicking on the "Dental Platform Milestones" document, located at the bottom of the far right hand column on this page.



Latest Research News
Year In Review 2015 ...
- Stemmed Implant Technology Inc ( SIT ) has successfully completed the fatigue/stress testing of the SIT dental implants. The testing was carried out at the Dental Research Institute, Faculty of Dentistry, University of Toronto under the ISO protocol for dental implants. The SIT dental implants are manufactured using additive manufacturing ( 3D metal printing ), in itself ground breaking for the dental implant industry. SIT wishes to express its appreciation to NRC’s IRAP program for the funding support through its Business Innovation Access Program ( BIAP ). August, 2015.
- SIT has successfully completed the stress and fatigue testing of its dental implants under the ISO 14801 standards. The testing was carried out at The Dental Research Institute, Faculty of Dentistry, University of Toronto. all implant sizes performed very well. Each implant size successfully completed 3 separate 5 million cycles as per required testing. July 31, 2015.
- SIT completes its second pre commercialization production of the SIT Implant at Mohawk College’s Advanced Manufacturing Resource Centre. May 25, 2015.
- SIT produces the implant placement tools using 3D plastic printing at Niagara College with financial support from NRC’s IRAP program. May 2015.
- SIT signs a financial support agreement with NRC’s-BIAP program for the stress and fatigue testing of the SIT dental implants – the testing will be carried out at the U of Toronto, School of Dentistry- May 2015.
- SIT successfully completes its first pre commercial production of the SIT dental implant using additive manufacturing ( 3D metal printing ) at Mohawk College’s Advanced Manufacturing Resource Centre. Partial funding was provided by NRC’s IRAP program. April 2015.
- SIT participated in the first “ Canada Makes: Additive Manufacturing Forum “ April 30th, 2015, Mohawk College, Hamilton Ontario, Canada.
- SIT’s President and CEO participated in a by invitation only roundtable discussion with the Minister of Science and Technology the Honourable Ed Holder – on issues that industry is facing for development and growth, and if the role of government initiatives is helpful. April 08, 2015.
- Niagara College in Niagara Falls becomes SIT`s college partner for quick prototyping, design and production; SIT is working closely with the College’s Advanced Manufacturing Research & Innovation Centre and has as its first project the production of the SIT dental implant placement process tools, which include: deep impression trays, moulds for acrylic guides and separation / alignment "T's". We are fortunate in having such expertise and capability close to our location.
- SIT has successfully obtained funding with Niagara College through the NRC-IRAP program to produce the SIT dental implant placement tools, which in themselves form part of a transformational dental implant placement process that is more patient, dentist and dental laboratory friendly and convenient.
- Mohawk College in Hamilton becomes SIT`s college partner for engineering and technology. The first project is the development of the additive manufacturing ( 3D printing ) parameters and production of the first batches of the industry disruptive SIT dental implant. SIT is working closely with Mohawk`s Additive Manufacturing Resource Centre, one of the most advanced centres of this type in North America.
- SIT has successfully obtained funding with Mohawk College through the NRC-IRAP program to develop the manufacturing parameters and production of the first run of the SIT dental implant.
- SIT meets with the Hon. Minister Reza Moridi to speak about the Mohawk college partnership and the development work with the Additive Manufacturing Resource Centre.
- PCT patent application for APPARATUS AND METHOD FOR PERFORMING IMPLANTS, files in January 2015 – important for the proper placement of Stemmed implants or regular screw type implants.


Year In Review 2014 ...
- SIT files a US provisional application for the Negative Implant, December 2014. An important technology for those that can not receive regular implants due to genetics, age, lifestyle or disease.
- SIT filed patent application for “Apparatus and Methods for Performing Implants" these products address the need for guided implant placement applicable for both the SIT Implant and conical screw type implants.
- SIT had meetings with Biomet 3i to introduce the SIT dental implant. Biomet 3i is one of the world’s leading dental implant companies.
- Meetings with Investors in the USA.
- Establishing MOUs with alliance partners (manufacturers/distributors), Whip Mix (Louisville Kentucky), Advance Precision (Boynton Beach, Florida), St. Catharines Patterns (St. Catharines, Ontario).
- First production run under ISO 13485-2013 standards Lab bench testing.
- Lab bench testing - fatigue testing of the production dental implant.
- Finishing touches put on clinical protocol.
- MOU developed with International Dental Arts, Guatemala City, Guatemala.
- Conducting pilot clinical trial, according to Best Clinical Practice protocols.
Year In Review 2013 ...
- SIT has held meetings with international investors representing interests from Europe, the Middle East, Asia and North America.
- A novel implant placement system was developed for SIT implants and other manufacturer’s conical implants to ensure the implant is in perfect position in the bone.
- Investor interest from Dubai, United Emirates, Singapore, China and India.
- SIT acquires HIT – a Buffalo New York company active in the dental products business for 35 years.
- Continued investment from Private investor community.
- Sales/Marketing agreements made with manufacturers and distributors in Canada and the USA.
- Participation in Medical Device Manufacturers conference.
- Formal development Health Canada/FDA clinical protocol.
Year In Review 2012 ...
- Development of a formal business plan.
- Presentations to private investors and companies representing private investors.
- Presentation at BioFinance on SIT technology.
- Meeting with Health Canada/FDA to formalize regulatory approval plan.
Professional Testimonials
- To give you and idea of the interest, we recently met with Dr. Christensen of Gordon J. Christensen Clinicians Report ®. A publication of CR Foundation. The group evaluates and reviews new products and technologies for the Clinician. After the meeting, Dr. Christensen said that:
"We were impressed with the concept and look forward to specific clinical procedures"
- Dr. Christensen
- We have also been meeting with McMaster University’s School of Medicine, who are interested in moving forward on the application of the technology to other joints in the body:
"Michael’s ( Lococo – SIT Founder ) idea is innovative and new on the fixation side of things. In order to provide a group of investors some idea of the concept we would have to provide diagrams in their application in total joints, in this case the hip. Additionally the biomechanics analysis being used in the dental application should also be available to promote this concept in other joint pathology."
- Robert Josefchak MD. FRCS(c) - Orthopaedic Surgeon, Regional Educational Leader Orthopaedic Surgery, Post Graduate Specialty Development Lead, Niagara Campus, Michael DeGroote School of Medicine, McMaster University, Hamilton Ontario
Dr. Michael Lococo

Founder
Chairman of the B.O.D.
mplococo@stemmedimplant.com
Direct: (905) 356-6000
Investor Brochure

Timeline